What Are the Licenses and Storage Requirements for Diagnostic Kits?
Table of Contents
Introduction
For healthcare MSMEs, small clinics, and pharmacies, the demand for diagnostic kits such as glucometers, rapid tests, and pregnancy kits has grown significantly.
These products help clinics expand services while creating new revenue streams.
But trading, storing, and selling diagnostic kits requires compliance with India’s medical device regulations.
This guide breaks down the licenses, storage standards, and record-keeping rules every small healthcare business should know before dealing in diagnostic instruments and kits.
Which Licenses Are Mandatory to Sell Diagnostic Kits in India?
In India, diagnostic kits are regulated as medical devices under the Drugs and Cosmetics Act and the Medical Device Rules, 2017.
To sell legally, suppliers and clinics may need the following licenses:
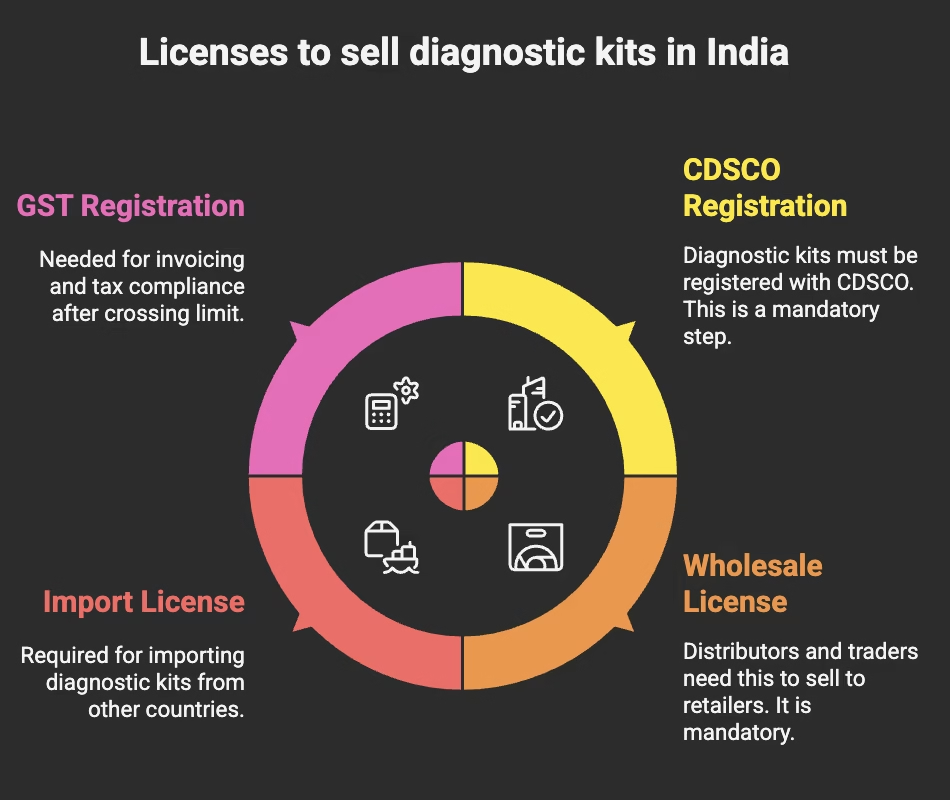
- CDSCO Registration
- All diagnostic kits (glucose monitors, rapid test strips, pregnancy kits, etc.) must be registered with the Central Drugs Standard Control Organisation (CDSCO).
- Wholesale License (Form MD-42)
- Mandatory for distributors, stockists, and traders who sell diagnostic instruments and kits to retailers or clinics.
- Import License (Form MD-15)
- Needed if you are importing diagnostic kits from abroad.
- GST Registration
- For invoicing and tax compliance, once the annual turnover crosses the prescribed limit.
Example: A diagnostic supplier in Chennai needed both MD-42 and CDSCO approvals to distribute COVID-19 rapid kits during the pandemic.
Do Small Healthcare MSMEs Need a CDSCO License for Handling Diagnostic Kits Like Glucometers or Rapid Tests?
The answer depends on the classification of devices:
- Class A & B Devices (Low/Moderate Risk):
- Items like glucometers, pregnancy kits, or rapid malaria test kits fall under this.
- Small clinics and pharmacies that only retail to patients generally don’t need a wholesale license but must purchase only from licensed distributors.
- Class C & D Devices (High Risk):
- More advanced diagnostic instruments (HIV, Hepatitis test kits) require stricter CDSCO oversight.
- Even retailers handling these may need regulatory checks.
Pro Tip for MSMEs: Always verify that your supplier is CDSCO-licensed so your clinic remains compliant even if you don’t hold the license yourself.
What Are the Storage Conditions Required for Diagnostic Kits in Small Clinics or Pharmacies?
Storage plays a crucial role in maintaining the accuracy and reliability of diagnostic kits:
- Temperature Control
- Most kits require storage between 2°C and–30°C.
- Use refrigerators for temperature-sensitive kits (e.g., ELISA kits, certain blood-based tests).
- Humidity Control
- Keep kits in dry conditions; high humidity can spoil reagents and test strips.
- Light Sensitivity
- Store away from direct sunlight to prevent chemical degradation.
- Stock Rotation (FIFO Method)
- Always sell older batches first to prevent expiry losses.
Example: A small clinic in Pune installed a dedicated mini-refrigerator for rapid dengue and malaria test kits to meet storage standards and pass inspection.
How Long Should Healthcare MSMEs Maintain Records of Diagnostic Kit Purchases and Sales?
Proper record-keeping ensures compliance and smooth audits.
- Mandatory Record Period: At least 2 years from the date of sale or distribution.
- Records to Maintain:
- Purchase invoices with supplier license details.
- Batch numbers, expiry dates, and stock movement registers.
- Sales receipts (especially if sold in bulk to other clinics or labs).
Inspection Readiness: CDSCO or state drug inspectors can request records anytime. Keeping digital logs (via the Vyapar app or Tally ERP) reduces paperwork hassles.
Where Can MSMEs Source Compliant and Certified Diagnostic Kits for Resale?
Sourcing from certified suppliers ensures both compliance and product reliability.
- Government-Approved Platforms:
- GeM (Government e-Marketplace) lists approved diagnostic instruments and kits.
- Authorized Distributors:
- Work only with CDSCO-licensed wholesalers.
- Direct Manufacturers:
- Cities like Ambala, Pune, and Hyderabad have certified diagnostic kit manufacturers.
- Reputed Online B2B Marketplaces:
- Medikabazaar, IndiaMART, and TradeIndia provide access to vetted suppliers at wholesale rates.
Pro Tip: Always demand a copy of the supplier’s MD-42 or CDSCO certificate before entering into bulk purchase agreements.
Conclusion
For healthcare MSMEs and small clinics, dealing in diagnostic kits can be profitable and patient-friendly, but only when backed by the right licenses, storage standards, and records.
Clinics don’t always need their own CDSCO license if they retail, but they must source from compliant distributors.
Safe storage, 2-year record retention, and verified sourcing protect your business from regulatory risk and ensure diagnostic accuracy for patients.
Frequently Asked Questions (FAQ)
Q1: Do all diagnostic kits need CDSCO approval in India?
A: Yes, every diagnostic kit must be registered with CDSCO before being sold.
Q2: Does a small clinic need a license to sell glucometers?
A: No, but they must purchase from licensed distributors and maintain invoices for records.
Q3: What storage is required for rapid test kits?
A: Store in cool, dry places (2°C–30°C) and avoid exposure to sunlight or moisture.
Q4: How long should records of diagnostic kit sales be kept?
A: At least 2 years, including supplier license details and batch records.
Q5: Where can MSMEs buy certified diagnostic kits?
A: From CDSCO-approved distributors, the GeM portal, or trusted B2B marketplaces like Medikabazaar.
Also Read,
- The Ultimate Guide: Working Capital Loans for Small Business (MSMEs) in 2025
Understanding the Impact of Payment Terms on Working Capital for Clinics
How Poor Inventory Management Hurts Working Capital in Pharmacies.
Want a Better Business Credit Score? Small Pharmacies Can Now Use UPI & Cards to Build It
Want a Better Credit Score? Use Small Daily Payments to Build Your CBIL (For Clinics & Pharmacies)
Case Study:How a Small Clinic Improved Its Working Capital Management